Third Law
Third Law

Most people don't know that there are three laws of thermodynamics. It turns out that the third law is very important to not only astronomical physics but also quantum physics. That's because it has to do with entropy, that elusive mysterious quality of not only all energy systems but also the universe as well.
I've already discussed entropy before and described it as the measure of order in a system. There is another more important aspect of entropy and that is the concept of absolute zero, and as you might know any time you have an absolute anything, people get their knickers in a twist.
The third law of thermodynamics is usually defined in this way: A perfect crystal at absolute zero has entropy of zero. When you start using the term 'perfect' you have a problem because there is no such thing as perfect.
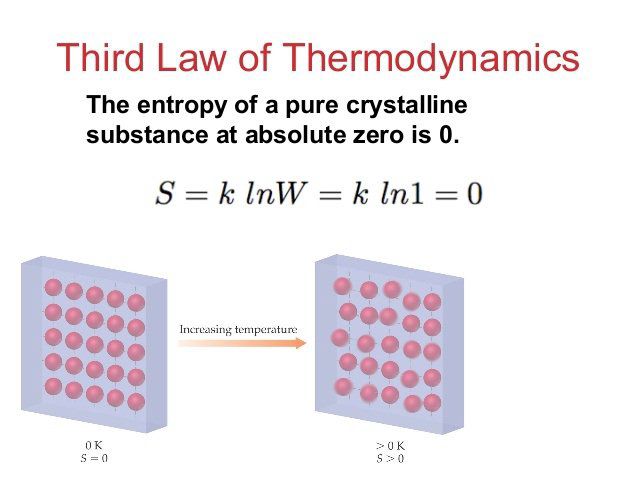
Quantum physicists have their own definition of the third law. They say it's when a quantum state is at its lowest energy state. They call it the ground state of minimal energy.
Essentially, if you cooled a gas to zero degrees Kelvin the molecules would cease moving. For a crystalline material the definition becomes more difficult. For one, the perfect crystal must be of a pure substance. Then, there is the problem of cooling it to absolute zero. Then there is the problem of actually measuring the temperature at absolute zero.
Well, it appears that the third law is true based on a paper in Nature Communications from researchers at Univeristy College London. They proved that it's impossible to cool as system to absolute zero with a finite number of steps. They call this idea the unattainable principle. They also proved that it's impossible to cool a system to absolute zero in finite time. In other words, it would require an infinite amount of time and an infinite number of steps. Infinite means that it's like a speed limit. At the present time, there is no equipment available to accomplish an infinite number of steps. It's like traveling at the speed of light. We're no way near accomplishing that.
To me, this is equivalent of trying to accomplish an Adiabatic process, which is a process that occurs without the transfer of heat or matter between a thermodynamic system and its surroundings. This is an ideal concept that would require an infinite amount of time to complete. In chemistry this is the gas law PV=nRT. This only works for an adiabatic system. In a way, the idea of absolute zero is much like that. It's an ideal concept that's unattainable.
Thanks for reading.
Bạn đang đọc truyện trên: AzTruyen.Top